Group Köhler
Phase-separated ubiquitination condensates – structure, function and turnover
Group Leader
Alwin Köhler
Alwin Köhler is the Professor of Molecular Biology and Scientific Director of the Max Perutz Labs. He received his doctoral training at Harvard Medical School, completed postdoctoral research at Heidelberg University, and became an independent group leader at the Perutz in 2010
- Institute Max Perutz Labs
- Phone +43 1 4277 61685
- Mail alwin.koehler@maxperutzlabs.ac.at
- Web https://www.maxperutzlabs.ac.at/koehler
Projects within consortium
We study how ubiquitination is spatially organized through phase-separated condensates on chromatin. By dissecting the assembly, function, and turnover of ubiquitination condensates, we aim to uncover new principles of ubiquitin signaling dynamics and how their dysregulation may impact genome regulation and disease.
Our research investigates a newly emerging mechanism of ubiquitin signaling: the formation of phase-separated ubiquitination condensates on chromatin. These dynamic, liquid-like compartments serve as specialized reaction centers, concentrating ubiquitin ligases and their substrates to stimulate site-specific ubiquitination during transcription.
We aim to understand how scaffold proteins and ubiquitin ligases cooperate to assemble these condensates, how condensate architecture and material properties influence ubiquitin transfer, and how chromatin-bound substrates are selectively targeted. Using genome-wide chromatin profiling and innovative single-molecule assays, we visualize condensate behavior in real time on DNA and chromatin templates, providing insight into the spatial regulation of chromatin-associated ubiquitination.
To reveal the molecular underpinnings, we employ an integrative structural biology strategy combining recombinant biochemistry, cross-linking mass spectrometry, cryo-electron microscopy, and mutagenesis. This will define the structure and surface properties of ubiquitination condensates and how they impact client protein recruitment and enzymatic activity.
Finally, we study how aberrant ubiquitination condensates are recognized and cleared, addressing an important but understudied aspect of ubiquitin-mediated proteostasis. As mutations in human scaffold proteins linked to condensate formation are associated with neurodevelopmental disorders, our findings may also offer new perspectives on the pathological consequences of defective ubiquitin signaling.
Through these approaches, we seek to establish fundamental principles governing the structure, function, and quality control of ubiquitination condensates.
Relevant literature
Polyansky AA, Gallego LD, Efremov RG, Köhler A, Zagrovic B (2023).
Protein compactness and interaction valency define the architecture of a biomolecular condensate across scales.
eLife. doi: 10.7554/eLife.80038
Gallego LD, Schneider M, Mittal C, Romanauska A, Gudino Carrillo RM, Schubert T, Pugh BF & Köhler A (2020).
Liquid-liquid phase separation directs ubiquitination of gene body nucleosomes.
Nature. doi: 10.1038/s41586-020-2097-z
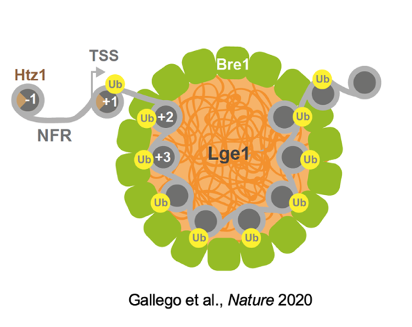
Model of a ubiquitination condensate. Lge1-Bre1 condensates form on active genes, creating dynamic reaction hubs for chromatin ubiquitination. NFR, nucleosome-free region; Htz1, histone variant.
Project members
-
Postdoc
Laura D. Gallego Valle
-
PhD student
América Ramírez Colmenero
-
PhD Student
Frane Miljkovic
-
Postdoc
Yap Shing Nim
-
Staff Scientist
Maren Schneider
Targeted Protein Degradation related publications by Group Köhler
- 2024 Seipin governs phosphatidic acid homeostasis at the inner nuclear membrane Nature Communications Go to publication →
- 2023 Lipid saturation controls nuclear envelope function Nature Cell Biology Go to publication →
- 2023 Protein compactness and interaction valency define the architecture of a biomolecular condensate across scales eLife Go to publication →